January 2009, Urologiia (Moscow, Russia: 1999)
G.G. Krivoborodov, E.B. Mazo, N.S. Efremov
Temporary stent – CoreFlow® Soft Stent – for diagnosis of the causes of incomplete bladder emptying in men with neurological diseases
The new device – CoreFlow Soft Stent – was used for diagnosis of causes of incomplete bladder emptying (IBE) in 19 men with neurogenic diseases. Group 1 consisted of 8 men with IBE and prostatic adenoma. Group 2 consisted of 11 men with IBE but no prostatic adenoma. The CoreFlow Soft Stent comprises an introducer and a stent. Different active lengths of the stent are available to match it to the length of the prostatic urethra. The stent has an anchoring balloon situated in the bladder and a second anchor located distally to the external sphincter. CoreFlow was introduced in a similar way as an ordinary Foley catheter. The bladder was filled with 200 ml of saline solution through the introducer and the stent.
The introducer part was then separated from the stent part positioned in the prostatic urethra. The stent part is connected to the integral ”pull-thread” device which runs through the urethra ending outside the meatus. Reposition of the stent using the special thread opens the striated urethral sphincter. Out of 8 patients with prostatic adenoma and neurogenic diseases 4 could urinate with the stent part positioned in the prostatic urethra indicating that prostatic adenoma was the cause for IBE.
These patients have undergone TUR. The other 4 patients of group 1 and 11 patients of group 2 could urinate only using Valsalva manoeuvre and after reposition of the stent for opening the striated urethral sphincter. This allowed us to conclude that these 15 patients suffered from detrusor underactivity. Our experience indicates that CoreFlow Soft Stent is a simple device for diagnosis of the causes of IBE in men with neurogenic diseases.
Objective
In a retrospective study the use of a new temporary stent – CoreFlow® Soft Stent-was evaluated in 51 patients. This new device was used instead of an ordinary indwellingcatheter post microwave treatment of BPH using theProstalund CoreTherm device
Method
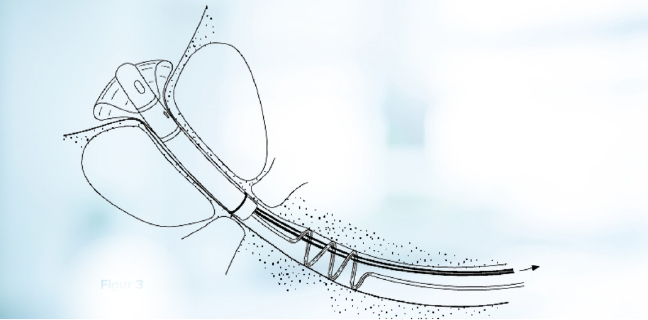
51 consecutive patients were included in the study. The device is shown in Figure 1. The device comes in different lengths of the stent part. The suitable length was determined using TRUS prior to the treatment. After the microwave treatment, the CoreFlow® was introduced in a similar way as an ordinary Foley catheter, see Figure 2. The bladder was flushed through the device and then filled with saline solution. The device’s rear part was then separated with the stent part positioned in the prostatic urethra and the ”pull-thread” and balloon tube running through the urethra and ending outside the meatus, see Figure 3.
After placement, the patients performed a voiding test to demonstrate:
■ sphincter controlled voiding function
■ continence
■ that the self-catheterization mechanism functioned
by forcing the externaL sphincter to open by using the
”pull-thread” of the device, see Figure 4.
Results
The CoreFlow® was found to be very easy to place and remove. One out of 51 patients needed to have the stent removed prematurely due to a blockage in the drainage canal. Most of the patients needed to slightly reposition the stent using the pull thread the first couple of days after the microwave treatment. Same patients also needed to use the ”pull-thread” to perform self-catheterization the first days after the procedure. No patients developed symptomatic UTI during the use of the stent. One patient had a positive urine culture after the removal of the stent.
Conclusion
The initial experience indicates that the device can successfully be used post microwave treatment to replace an indwelling catheter. The CoreFlow® seems to have a large potential in lowering the risk of UTIs post invasive procedures like microwave therapy as well as improving health related quality of lite aspects.
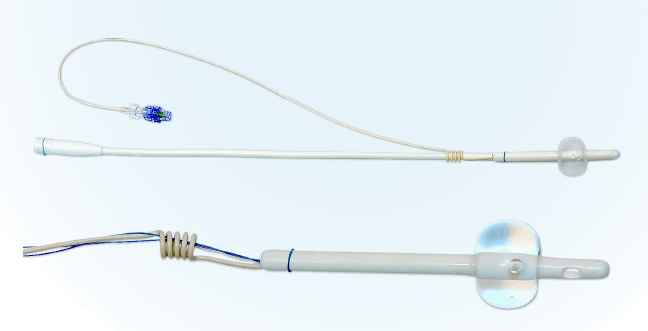
Figure 1. Pictures of CoreFlow® Soft Stent. The top picture shows the device prior to separation of the lower part. The bottom picture shows the upper – i.e. the stent part of the device. The stent is anchored at the bladder neck with the balloon and below the external sphincter with the coil.
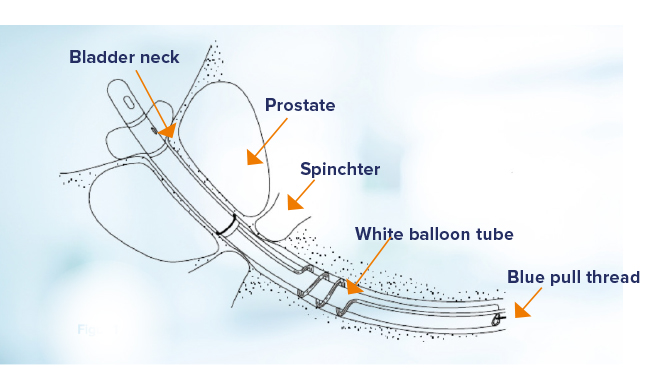
Figure 2. Schematic showing the CoreFlow® during insertion. lnserted as an ordinary Foley catheter.
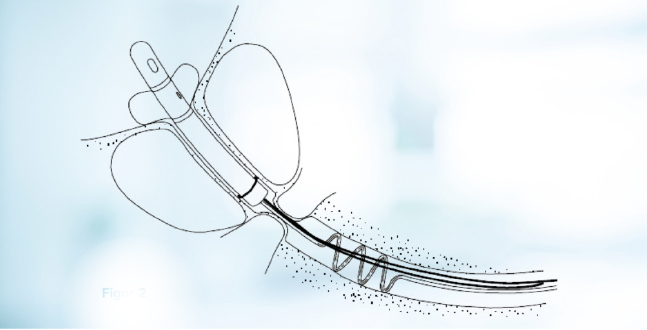
Figure 3. Sphincter controlled voiding is possible with CoreFlow® in place as a stent in the prostatic urethra.
Figure 4. Self-catheterization using the ”pull-thread” mechanism. The balloon is made of a soft material allowing it to be compressed when force is applied. The rear part of the stent thereby opens a flow channel past the sphincter. When the ”pull-thread” is released the stent is repositioned by the balloon returning to it’s original shape.
Temporary stent – CoreFlow® Soft Stent – for diagnosis of the causes of incomplete bladder emptying in men with neurological diseases
January 2009, Urologiia (Moscow, Russia: 1999)
Authors: G.G. Krivoborodov, E.B. Mazo, N.S. Efremov
Read the complete study here
Retrospective pilot study evaluating a new device, the Coreflow® Soft Stent
Sonny Schelin, Specialistläkargruppen, Kalmar
Download the study in PDF Retroperspective Pilot study_ Schelin